Independent Report – A newly established CDC vaccine panel of advisers in the United States. Appointed by Health Secretary Robert F. Kennedy Jr., is set to meet soon to discuss several important vaccination topics. Among the key issues on the agenda is the evaluation of flu vaccines that contain a mercury-based preservative known as thimerosal. This meeting, scheduled for June 25 and 26, will also include discussions about recommendations for the measles vaccine. Particularly concerning its administration to young children.
The panel serves as advisers to the Centers for Disease Control and Prevention (CDC) and plays a critical role in shaping public health policies related to vaccines. Their upcoming meeting will address who should receive newly developed vaccines for respiratory syncytial virus (RSV) and influenza. RSV is a common respiratory virus that can cause severe illness, especially in infants and older adults. As vaccine development progresses, the panel’s guidance on its use will be crucial.
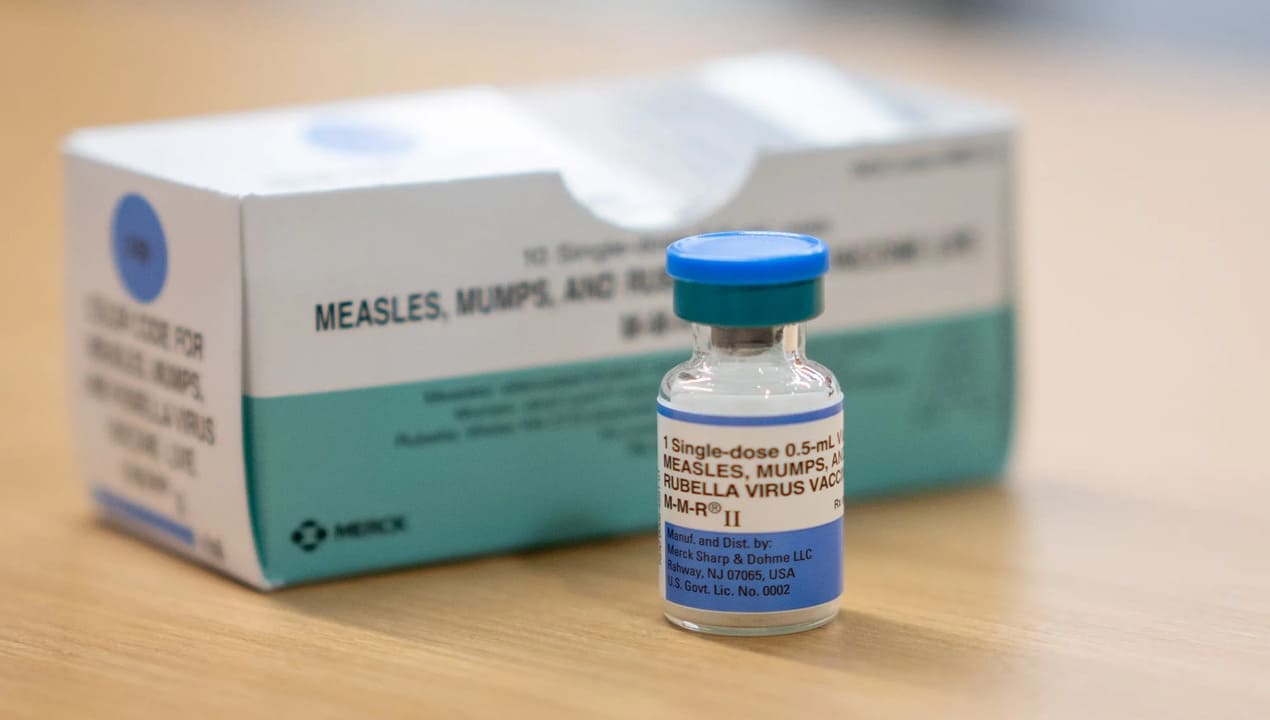
One of the significant points of discussion will focus on whether children under five years of age should receive a combined vaccine that protects against measles, mumps, rubella, and varicella, known as the MMRV vaccine. This combined shot aims to provide immunization against four different diseases in a single injection, which could help improve vaccination coverage among young children. However, the panel will carefully examine the benefits and any potential risks associated with administering this vaccine to very young children.
Also Read : Mike Lindell Ordered to Pay $2.3 Million for Defaming Dominion
The agenda released by the CDC does not specify who will present information or data regarding the MMRV vaccine or thimerosal during the meeting. Nevertheless, it is expected that experts and researchers will share their insights to assist the panel in making informed decisions. Thimerosal has been a topic of debate for many years due to concerns about its mercury content. This preservative has been used in multi-dose vaccine vials for decades in the United States to prevent contamination and ensure vaccine safety.
Despite concerns, the CDC has maintained that thimerosal in vaccines is safe at the low levels used. It is important to note that thimerosal is not present in most single-dose vaccine vials. And also many vaccines have been produced without it to address public concerns. However, flu vaccines often still contain thimerosal, especially those packaged in multi-dose vials. Which makes this discussion particularly relevant as the panel evaluates whether these vaccines should continue to be recommended.
The upcoming meeting of this vaccine advisory panel marks a significant moment as it will be the first gathering of advisers appointed by Robert F. Kennedy Jr. Since assuming office, Kennedy has taken a notably different approach to vaccine policy compared to previous health officials. Which has raised interest and attention within the public health community. His appointments and the panel’s decisions may influence the direction of vaccine recommendations in the coming years.
The panel’s recommendations are highly influential because the CDC often adopts their guidance when making official vaccination policies for the country. These policies affect millions of children and adults who rely on vaccines for protection against infectious diseases. Therefore, the deliberations regarding the MMRV vaccine and flu vaccines containing thimerosal will be closely watched by healthcare providers, parents, and public health officials alike.
Vaccination remains one of the most effective tools in preventing outbreaks of contagious diseases such as measles, mumps, rubella, varicella, influenza, and RSV. Each of these illnesses can cause serious health complications, especially in vulnerable populations like young children, the elderly. And also those with weakened immune systems. Ensuring safe and effective vaccines are available and recommended to appropriate groups is a top priority for public health agencies.
As this new vaccine advisory panel begins its work, it will likely review the latest scientific data, clinical trial results. And also safety information related to the vaccines under consideration. Their goal is to provide clear, evidence-based recommendations that balance the benefits of immunization with any potential risks. The upcoming meeting will be an important step in continuing efforts to protect public health through vaccination.
In summary, the U.S. vaccine advisory panel appointed by Health Secretary Robert F. Kennedy Jr. is preparing to vote on flu vaccines containing thimerosal and discuss recommendations for the MMRV vaccine for children under five. The meeting will also cover guidance on vaccines for respiratory syncytial virus and influenza. While details about presentations on these topics remain unspecified. The decisions made by this group will have a significant impact on future vaccination policies. The discussions reflect ongoing efforts to maintain vaccine safety and effectiveness for the protection of the population.
Also Read : The Rise of Soulful Listening: Spiritual Podcasts Explained